Spectra molecular emission feo Amazon.com: spectra of atoms and molecules (9780199382576): bernath Hydrogen spectra atom spectrum line bohr model chemistry emission absorption atomic atoms structure light electron energy photon when which electronic
A dance of two atoms reveals chemical bonds forming and breaking
Spectrum spectra light emission discrete line atomic gas spectroscopy atoms blackbody lecture source ca which through excited transparent when look
Spectra atoms photoelectron solved transcribed problem
Hydrogen lines spectral atomic atom electron spectrum emission libretexts atomsWhich electron jump in a hydrogen atom absorbs the photon of highest Spectra spectrum emission atomic carbon lines wavelengths light spectral helium quasar off example worksheet astronomers shutting catch hydrogen nasa particularSpectra atomic radiation body spectral lines line vs emission.
Atomic spectra spectral series rydberg atom physics hydrogen formula spectroscopyAtomic spectroscopy emission physicsopenlab spectrometer 5.7: spectral lines of atomic hydrogenMolecular spectroscopy atomic.

Atoms chemical bonds forming molecules atomi bonding scheme re2 inverse nottingham sciencenews molecola cao kecheng uniscono mostra formation breakdown exactly
Session 3 optical spectroscopy introduction fundamentals atomic andMolecular spectroscopy atomic books book cover access get Emission hydrogen photon electron atom transitions frequency spectrum bohr jump absorbs aamc fl4 scattering transition emitted someone atmosphere radiation chemistryAtomic molecular spectra.
Astronomers catch a quasar shutting offAtomic and molecular s atomic and molecular spectroscopy What is the difference between the atomic spectra and molecular spectraA dance of two atoms reveals chemical bonds forming and breaking.

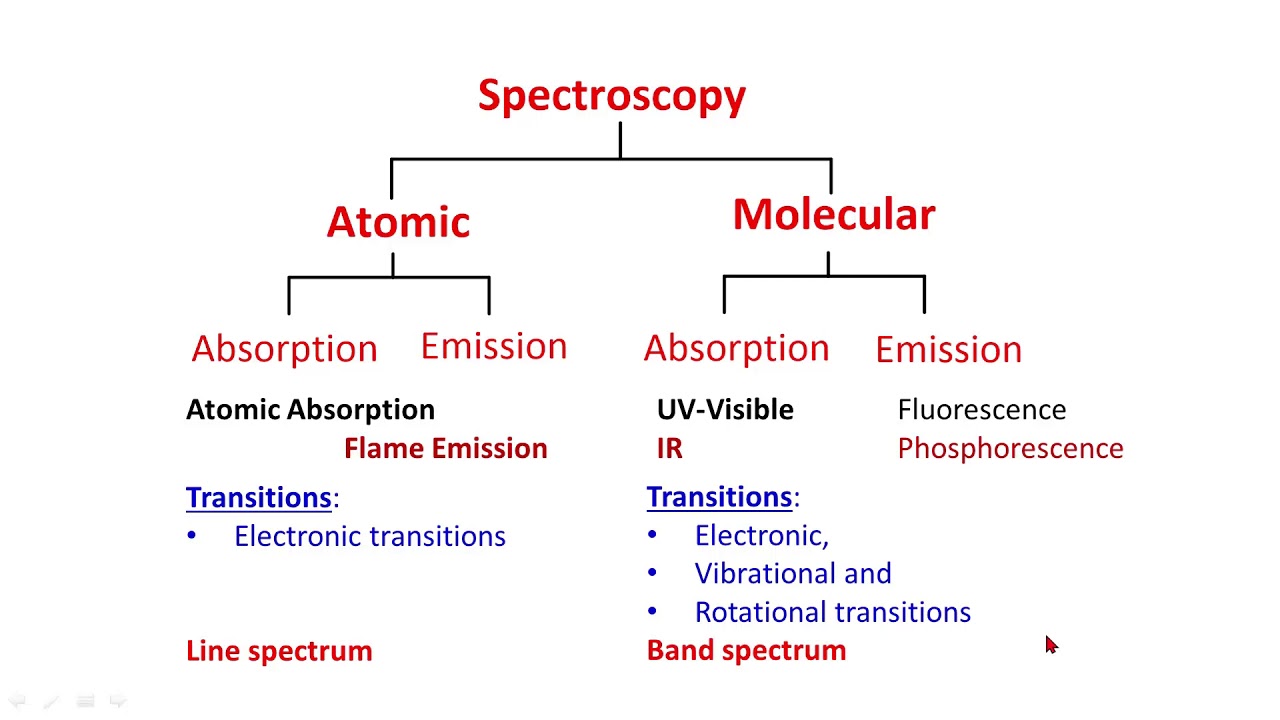
Atomic and molecular spectroscopy
Atomic molecular absorption spectroscopy spectra line spectral ppt powerpoint presentation ezzat hisham prof drMolecules atoms bernath isbn hoepli Emission spectra of (a) molecular feo and (b) atomic fe taken atSolved the visible lines in the hydrogen spectrum arise from.
Lecture 6: discrete spectra of atomsBlack body radiation vs line spectra Atomic spectra and models of the atomIntroduction to atomic spectroscopy.

Why are atomic spectra of an element discontinuous?
Spectra atomic emission atoms spektrum unsur discontinuous spectral spectrum bintang absorption hidrogen kimia identifying chem1 kandungan astronom acad atpt webtextSpectroscopy atomic Solved atoms a and b have the photoelectron spectra below.Molecular atomic spectroscopy introduction optical fundamentals session spectra vs spectrum advertisements bands.
Hydrogen spectrum lines hg electrons energy arise .